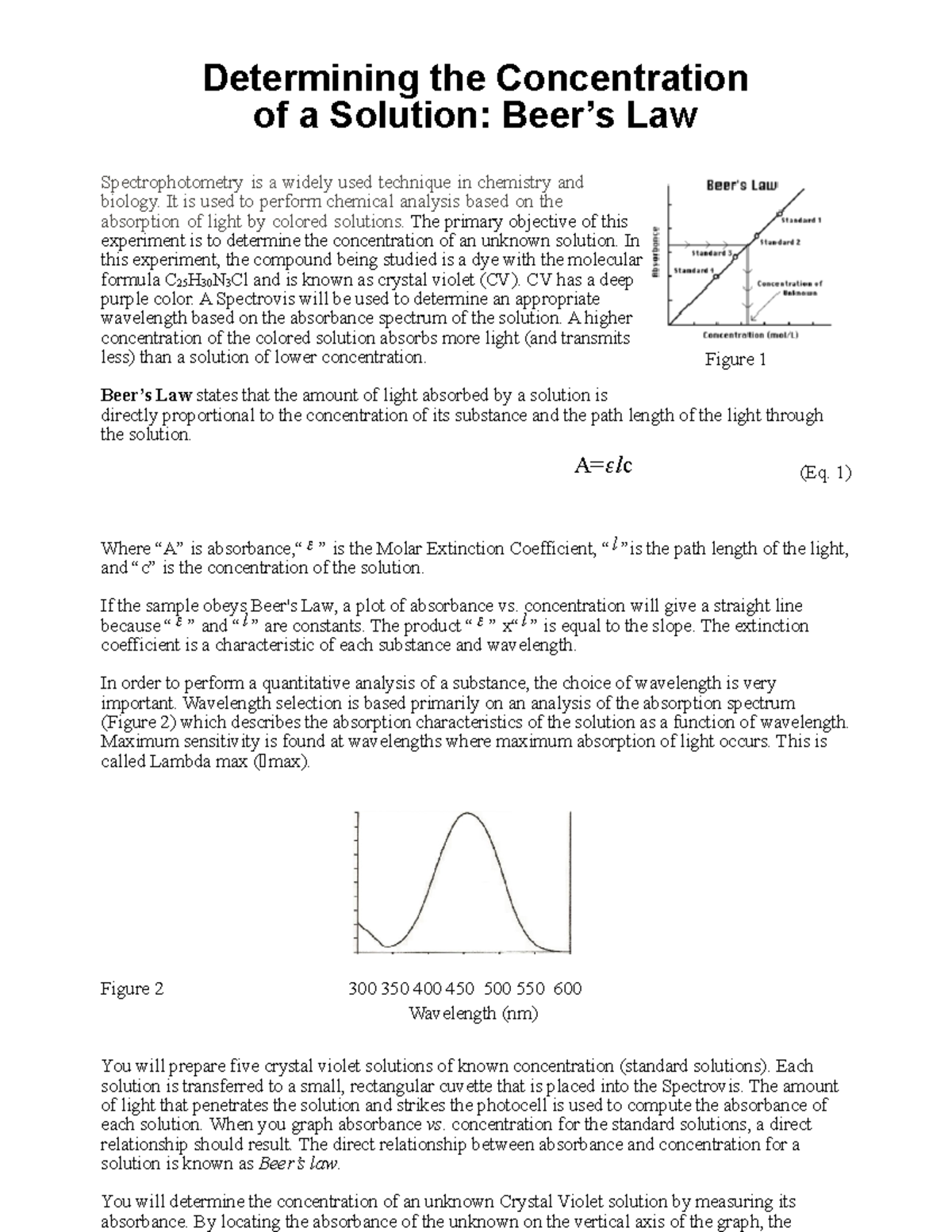
Beer's Law Biochemistry . beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of polymer degradation. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and.
from www.studocu.com
the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of polymer degradation. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium.
Chem 181 4 Beer's Law Beer Lab Pre lab Determining the
Beer's Law Biochemistry since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of polymer degradation. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following.
From www.slideserve.com
PPT SURVEY OF BIOCHEMISTRY Amino Acids and Proteins PowerPoint Beer's Law Biochemistry in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of polymer degradation. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. the two laws,. Beer's Law Biochemistry.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Biochemistry the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of polymer degradation. the amount of light that a species absorbs in a spectroscopic transition can be related. Beer's Law Biochemistry.
From chemistrysources.com
قانون بيير (بير) Beer's Law التعريف و المعادلة مصادر الكيمياء Beer's Law Biochemistry the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of polymer degradation. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer's Law Biochemistry.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Biochemistry since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. in chemistry, beer’s law finds solution concentration and helps assess oxidation and. Beer's Law Biochemistry.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Biochemistry the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy. Beer's Law Biochemistry.
From www.slideserve.com
PPT Exp 14B Determining an Equilibrium Constant PowerPoint Beer's Law Biochemistry the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. since the concentration, path length and molar absorptivity are all. Beer's Law Biochemistry.
From dxoccvohi.blob.core.windows.net
Beer's Law Path Length at John Lee blog Beer's Law Biochemistry the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of polymer degradation. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. the. Beer's Law Biochemistry.
From chemistnotes.com
Beer lambert law Derivation, deviation, application, and limitations Beer's Law Biochemistry the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. in chemistry, beer’s law finds solution concentration and helps assess oxidation and. Beer's Law Biochemistry.
From studylib.net
Beer`s Law Beer's Law Biochemistry since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of polymer degradation. the two laws,. Beer's Law Biochemistry.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer's Law Biochemistry the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of. Beer's Law Biochemistry.
From openlab.citytech.cuny.edu
Beer’s Law Biology 1101 Course Hub Beer's Law Biochemistry in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of polymer degradation. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law Biochemistry.
From ecency.com
Spectrophotometry Simplified The BeerLambert Law in Spectrophotom... Beer's Law Biochemistry in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of polymer degradation. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law Biochemistry.
From www.youtube.com
Beer's Law Concept, Calculations, & Lab YouTube Beer's Law Biochemistry in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of polymer degradation. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Beer's Law Biochemistry.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Biochemistry the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. the amount of light that a species absorbs in a spectroscopic transition. Beer's Law Biochemistry.
From www.studocu.com
Biochemistry revision notes Biochemistry The BeerLambert law Beer's Law Biochemistry since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of polymer degradation. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Beer's Law Biochemistry.
From www.youtube.com
BEERLAMBERT LAW GATE CHEMISTRY/BIOCHEMISTRYNumerical Solutions Beer's Law Biochemistry the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of polymer. Beer's Law Biochemistry.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Biochemistry the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. in chemistry, beer’s law finds solution concentration and helps assess oxidation and the rate of polymer degradation. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing. Beer's Law Biochemistry.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer's Law Biochemistry the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. beer’s law, in spectroscopy, a relation concerning the absorption of. Beer's Law Biochemistry.